Preprint from Veterinary Clinics of
Fluid Therapy in the Neonate – NOT Your Mother’s Fluid Space!
Jonathan E. Palmer, VMD, DACVIM
Associate Professor of Medicine, New Bolton Center, University of Pennsylvania and Director of Neonatal/Perinatal Programs, Graham French Neonatal Section, Connelly Intensive Care Unit, New Bolton Center, University of Pennsylvania
J.E. Palmer, VMD
New
(610) 444-5800 ext 2412
FAX (610) 925-8100
Synopsis: The neonatal fluid space is unique. High capillary filtration rate and increased interstitial space both serve to supply readily available fluid in times of need and, in part, are responsible for the neonate’s inability to easily deal with fluid overload. Critical neonates also have a variable sodium balance which may require sodium restriction in some cases or result in sodium wasting in others. In addition, critical neonates have a high energy demand which should be met by a constant supply of exogenous glucose. Special attention to the volume and type of fluids used is required to meet, but not exceed, the neonate’s fluid, energy and sodium needs.
Fluid therapy is one of the most commonly utilized therapeutic modalities in critical care. Water is the milieu of life. All cellular functions occur in water and depend on proper osmolality. With few exceptions, cells only survive if surrounded by a fluid reservoir in the form of the interstitium. Fluid balance including total body water and interstitial and cellular osmolarity are very important in maintaining cellular health. When normal physiology is not overly disrupted, when there is adequate renal function, gross errors in fluid therapy are forgiven. However, when renal function is severely compromised, as is true in many critical neonates, or when the special limitations the neonate must deal with during the transition from fetal physiology are ignored, mistakes in fluid therapy can have devastating consequences. As with all therapeutic interventions, fluid therapy should be based on an understanding of the patient’s physiology and an educated guess about the pathophysiology occurring at the time. No one fluid therapy plan will be suitable for all cases. Fluid therapy should be tailored to the individual rather than having one fluid plan forced on all cases. However, the starting point of the fluid plan should be universal so that gross errors in formulation are avoided. This article will outline a fluid plan based on neonatal physiology which can form the starting point in formulating fluid therapy for the critical neonate.
Fetal
and Neonatal Fluid Physiology
The neonatal period is a dynamic transition between fetal and pediatric physiology. Thus to understand fluid balance in the neonate, an understanding of the fluid physiology of the fetus and the dynamic changes that occur during the neonatal period are essential. There are major differences between the fetus and adult in total body water, interstitial fluid dynamics, rates of fluid movement through the interstitium and the self-contained nature of the fetal fluid system. [1,2,3] Perhaps the most important difference in physiology involves the dynamic nature of the fetal/neonatal capillary epithelial barrier and its effect on plasma and interstitial fluid volume dynamics. [4]
Fluids are abundant in the fetus. Fluid accounts for over 90% of the fetal body weight through the first half of gestation. There is a gradual decrease in fluid content of the fetus during gestation primarily due to a decrease in extracellular fluid. Still, near birth 75-85% of the body weight is water, depending on the species. [1,2] The dynamics of extracellular fluid distribution is different in the fetus. The distribution of fluid between plasma and interstitium depends on the balance between lymphatic function and capillary permeability and the characteristics of the interstitium. The fetal interstitium contains more ground substance and is very compliant allowing it to hold large amounts of fluid without generating increased interstitial pressure thus the added fluid is not free fluid (edema). Fetal capillaries have increased permeability characteristics. In fetal lambs the capillary filtration coefficient is five times greater than adult values and permeability for plasma proteins is 15 times greater than in adults. [3] Much of the increased capillary filtration rate is driven by increased capillary pressure. [5] In the fetus and initially in the neonate, precapillary tone is poorly developed. This allows transmission of systemic pressures to the capillaries, increasing filtration pressure.
To balance the increased capillary filtration, lymph flow rate is much greater in the fetus. [6] Volume expansion of the fetus will increase capillary filtration above the high basal rate producing more lymph. Fetal lymph flow has the ability to increase to meet this challenge and keep the interstitial fluid volume stable. [2] At the same time, the critical venous pressure that will begin to impede lymphatic return is close to normal venous pressure. [7] The consequence of this is that even small increases in venous pressure, such as may occur with intravenous fluid therapy, may result in significant retention of interstitial fluid. There are complex, poorly understood endocrine and autonomic controls of lymph production. [5]
Changes in fluid balance in preparation for the fetal transition begin days before birth. Significant volumes of amniotic and allantoic fluids are shifted to the fetal body associated with changes in catecholamine, vasopressin and cortisol levels. [3] Concurrently there is an increase in blood pressure by 20% which is transmitted to the capillaries resulting in a shift of these fluids as well as protein into the interstitium. [5] During labor there is a further loss of plasma volume into the interstitium secondary to increases in systemic blood pressure causing an increase in capillary leak. The increase in systemic blood pressure is caused both by physical pressure on the fetus during labor and an increase in vasoactive hormones such as vasopressin, norepinephrine and cortisol. [5]
Disease states will modify these fluid shifts. If hypoxia is an important component, it will amplify the loss of plasma volume into the interstitium. Mild hypoxia will result in increased capillary permeability, probably through increased filtration pressure. Severe hypoxia is associated with increased arterial/venous pressures which are likely transmitted to the capillary beds. There may also be significant translocation of fetal blood from the placenta to the fetal body causing a further increase in capillary pressure. [5] The increased capillary pressure will increase capillary filtration and further decrease plasma volume. [8]
Both the physiologic and pathophysiologic response results in an increase in interstitial fluid volume. This has important adaptive consequences. Take for instance the response to hemorrhage in the fetus or neonate. Neonates are at high risk of hemorrhage from umbilical structures. The expanded interstitial space serves as a reservoir for both fluid and protein in response to acute hemorrhage. After acute hemorrhage in adults, 24 to 48 hours is required for full return of plasma volume when not treated. The response is much more rapid in the fetus. First of all, a higher proportion of blood must be lost before there is a significant decrease in blood pressure because of the very rapid mobilization of interstitial fluid. Second, the return to normal plasma volume is much more rapid. After the loss of 30% of the plasma volume over 2 hours, the fetus will have restored two times the amount which would occur in an adult within 30 minutes and the total blood volume will be normal within 3-4 hours (about 1/10 the time required for the adult). [9,10] This is mediated by translocation of fluid and protein from the interstitial reserves. This response is not only present in the fetus but also the neonate during the first week, at least in lambs. [2,5]
Differences can also be seen in the response of the fetus and neonate to isotonic fluid loading. In adults between 20-50% (dependent in part on state of hypovolemia and dehydration) of an isotonic fluid load is retained in the intravascular space 30-60 minutes after infusion. In the fetus only 6-7% will be retained. [11] The neonate, in the transition state between fetus and adult, has intravascular fluid retention in between these two extremes but approaching the adult value. The poor intravascular retention has to do with capillary filtration coefficient and high interstitial to vascular compliance ratio. High capillary filtration coefficient results in rapid fluid movement across the capillary. The interstitial to vascular compliance ratio allows more extensive fluid movements without resistance. So, vascular fluid expansion by crystalloid infusion results in a transient increase in capillary pressure, which in turn, allows for rapid redistribution of the fluid. [12]
This same phenomenon has important implications in the neonate's inability to compensate for increased fluid loads. In the normal adult, when a fluid load is administered, it is rapidly excreted via the kidneys. This, in part, is due to the increased vascular volume modulating vasopressin, renin and atrial natriuretic peptide levels resulting in a diuresis. This does not occur in the neonate. [13] The rapid redistribution of fluids with low intravascular retention allows little stimulus for changes in vasopressin or rennin levels. Atrial natriuretic factor only transiently increases. As a result increased urine flow is very transient and most of the fluid load is retained long-term. [5,14] Neonates, especially ill neonates, retained fluid loads a long time and thus do not handle large fluid loads well.
These physiologic differences have therapeutic implications. The neonate is in a state of constant change. Any individual’s response will depend on the state of maturation of the capillary membrane and precapillary tone. Because of the interstitial reserve built into the neonate, the neonate can respond more rapidly and completely than the adult to hypovolemic challenges by rapid mobilization of these reserves. They are better able to maintain volemia. However the corollary is that once distress is detected in the neonate they are in real trouble. Neonates have a low-pressure vascular system. [15] The low systemic blood pressures may be important in maintaining plasma volume. Any increase in arterial pressure, if transmitted to the capillary, will decrease vascular fluid volume and cause increased protein leak. The presence of this response depends on the state of maturation but probably is present at least for the first five to seven days of life. [5] During this period, attempts to increase blood pressure may actually decrease volemia. Giving large volumes of crystalloids will result in little retention in the intravascular space. Giving colloids may not help since they will also leak into the interstitial space. When giving parenteral fluids, the neonate will tend to become fluid overloaded both because of its inability to handle fluid loads secondary to this physiology and because of the limitations of renal function. Once overloaded with fluids, prolonged retention should be expected. Colloids may exacerbate the retention of fluids by holding fluid in the interstitial space. All of these responses depend on the state of maturation of precapillary tone and the epithelial cell and the presence of epithelial damage which may occur with hypoxic insults or sepsis. Such pathologic states may delay maturation and result in the failure of the neonate to make the transition to a more mature fluid handling ability.
Sodium handling is rather unique in neonates. During fetal development, with immature renal tubules lacking the density of sodium transporters, there is a high fractional excretion of sodium. [16-18] This rapidly changes during the fetal transition. Near birth the neonate becomes very efficient in conserving sodium as manifested by a low urinary fractional excretion. [17,18] This conservation has very important survival advantages. The neonate requires at least 1 mg/kg/day for growth (primarily forming more interstitium and for bone matrix). Yet at the same time, its diet, fresh milk, is sodium poor. Mares milk has a sodium level of approximately 9 – 12 mEq/l. [19] So a foal drinking 10 to 20% of its body weight in milk will only ingest 0.9 to 2.4 mEq Na/kg/day.
There are several mechanisms of sodium conservation which are unique to the neonate which allow it to thrive on a low sodium diet. [16] But, unlike the adult who modulates distal tubule sodium absorption to achieve sodium balance, the neonate’s renal sodium conservation mechanisms continue to function efficiently in the face of excessive sodium intake. [17,20,21] Thus the neonate is prone to sodium overload and has difficulty balancing sodium, especially when treated with sodium based crystalloids. Sodium handling is further confused when the neonate suffers a hypoxic ischemic event. These foals often have renal tubular damage and may begin to develop significant sodium wasting. Without measuring renal sodium excretion or alternatively sodium fractional excretion, it may be difficult to tell if the critical neonate requires sodium restriction because it is following the normal sodium conserving physiology or if the foal needs supplemental sodium because of pathologic renal sodium wasting. Sodium overload will lead to fluid retention with expansion of the interstitial fluid volume manifested as edema which may interfere with normal organ function and perfusion. Sodium wasting will lead to decreased osmolarity, intracellular fluid shift leading to cellular edema and possible serious neurological consequences. Both extremes must be avoided when formulating a fluid therapy plan.
Fluid
therapy
There are several indications for intravenous fluid therapy. Crystalloids or colloids are often given for volume repletion of the ECF to correct hypoperfusion caused by hypovolemia. Crystalloids are commonly used to correct dehydration (normalize ICF) as a means of cellular resuscitation to replace pathologic losses or to meet maintenance fluid needs caused by physiologic losses. Whole blood may be given to treat anemia. Plasma may be given as a colloid to restore oncotic pressure in the treatment of hypovolemia, to increase buffer base, as immunotherapy, for coagulation normalization or for cellular nutrition. This discussion will focus on the use of fluids to insure volemia, insure hydration and meet maintenance needs of water, sodium and energy.
Fluid therapy for hypovolemia
Hypovolemia, as occurs in severe sepsis, septic shock, high volume diarrhea or acute hemorrhage, demands immediate therapeutic intervention. When giving large volumes, it is important to use fluids with a normal strong ion difference (Table 1). Using large volumes of fluids with no strong ion difference, such as saline, will have a significant acidifying effect and should be avoided. On the other hand, treatment with balanced ionic solutions such as Normosol®–R, Plasmalyte® or lactated ringers solution will tend to normalize the strong ion difference. Fluid boluses of 20 ml/kg over 10 to 20 minutes with re-evaluation of perfusion after each bolus are most efficient. [22] Evidence of successful therapy include improved pulse quality, warm legs, low core to peripheral temperature gradient, return of borborygmi, urine production, improved mental status and improved blood pressure. Repeat fluid boluses are usually required. If greater than 60-80 ml/kg is required, inopressor therapy, such as dopamine, dobutamine, vasopressin or norepinephrine should be initiated. [23] Return of adequate perfusion may require up to 200 ml/kg during the first 1 to 2 hours of treatment. Checking perfusion status after each 20 ml/kg dose helps limit the amount of fluids given to only the amount required to satisfy the immediate needs, avoiding excessive fluid overload. If ongoing losses continue, such as with diarrhea or polyuria, these losses should be replaced by normal strong ion difference sodium based fluids. The ongoing losses should be estimated and replaced in addition to the maintenance fluids calculated below.

Fluid therapy for glucose support
All compromised neonates will benefit from exogenous glucose support. Supplying energy in the form of glucose will decrease the need for catabolism. This allows the foal’s metabolic energy to be focused on recovery and not support. It is important to remember that the blood glucose level is not like a “gas gauge.” The level of blood glucose does not relate directly to adequate glucose stores. Rather blood glucose levels are a summation of glucose mobilization and utilization. The hypoglycemic individual is utilizing glucose faster than it is mobilized. The normoglycemic individual has well balanced mobilization and utilization. The hyperglycemic individual is mobilizing glucose faster than it is being utilized. In all 3 situations there may or may not be adequate glucose stores. In all 3 situations the individual will benefit from exogenous glucose support.
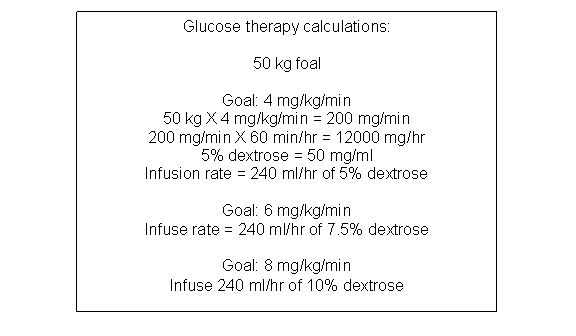
The goal of glucose therapy in the neonate is to reverse catabolism by supplying glucose at a rate equal to that supplied by the placenta before birth. Placental glucose transfer rates vary between 4 and 8 mg/kg/min in most species. The actual rate varies between species and varies with energy intake of the dam. The normal rate for the fetal foal is 6.8 mg/kg/min where as for the fetal calf it is 5 mg/kg/min. [24] Initially the neonate can be begun on 4 mg/kg/min and if this is tolerated without hyperglycemia the rate can be increased to 6 mg/kg/min and finally 8 mg/kg/min. The rate of increase will depend on the initial blood glucose level (if extremely low, the increase should proceed rapidly) and the foal’s metabolic response to the exogenous supply. The response to exogenous glucose support varies with the neonate’s physiologic state. Normal neonates adapt readily to exogenous glucose therapy but neonates with perinatal challenges may not. Compromised neonates may become (or remain) hyperglycemic due to a delayed insulin response, continued glucogenesis despite the exogenous source or stress glucogenesis. Other compromised neonates may become (or remain) hypoglycemic secondary to a SIRS response or other reason for hypermetabolism. Foals with severe sepsis/septic shock may require glucose infusion rates as high as 20 mg/kg/min to meet metabolic demands. Some compromised neonates appear to have metabolic anarchy with complete failure of metabolic transition from the fetal state. These foals experience metabolic confusion. With high exogenous glucose loads the addition of thiamine to the fluids may help ensure proper metabolism.
Foals which become hyperglycemic while being treated with exogenous glucose will benefit from regular insulin therapy. [25] Continuous infusion of regular insulin is well tolerated by most neonates, allowing more control of glucose kinetics. Most cases appear insulin deficient and not insulin resistant. Thus they respond to very low insulin infusion rates in the range of 0.00125 - 0.05 U/kg/hr, even in the face of sepsis. The requirement of low levels of insulin reflect slow adaptation to regulation, one aspect of a condition I refer to as neonatal metabolic maladaptation.
Fluid therapy for Maintenance Requirements
The volume of fluid required to maintain fluid balance depends on many variables. These include metabolic rate, the degree this metabolism is supported by catabolism, insensible water loss and most importantly surface area to mass ratio. Metabolic rate varies with surface area to mass ratio and with pathologic condition. Catabolism is important because it will increase the osmotic load which needs to be excreted (requiring water) even though it results in production of a small amount of metabolic water. The volume of insensible water loss will depend on the ambient humidity and temperature, respiratory rate as well as surface area to mass ratio. Other important variables include gastrointestinal water loss and urine osmotic load which will be increased by many medications, with hyperglycemia and with catabolism and the ability of the kidneys to concentrate this osmotic load (the amount of water required to excrete the osmotic load). Because all of these variables differ from one individual to another, there is no “correct” maintenance fluid rate. Thus the clinician must guard against being locked into a fluid rate based on tradition, but rather should seek a starting rate that suits most cases with the willingness to be flexible guided by the presents of confounding influences.
I have found that using the Holliday-Segar formula [26, 27], developed in 1957, I can meet the maintenance needs of most neonates but at the same time, avoid fluid overload. This formula works well for neonates weighing anywhere from 1 kg to 100 kg, making it ideal for neonates of all species. The formula calculates a “dry” fluid rate for most foals, which I feel is preferred. In most critical neonates fluid overload is more of a problem than mild fluid restriction once volemia has been insured. This is especially true in the patient who has renal compromise. Partially restricting fluids will spare the compromised kidneys the extra work of excretion of unnecessary fluids. In the Holliday-Segar philosophy it is acknowledged that maintenance fluid needs are related to basal metabolism. Metabolism produces heat which must be dissipated by insensible evaporation and solute byproducts which must be excreted in the urine. [27] Insensible losses and urinary losses are the 2 major sources of basal fluid requirements. Basal metabolic rate is higher per kilogram in neonate and higher per kilogram in smaller animals primarily because of variation in body size, thus mass to surface area ratio. In fact basal metabolic rate and thus basal fluid needs can be estimated based on surface area. The Holliday-Segar formula, developed to estimate basal metabolic rate based on body weight, also estimates fluid needs. [26, 27] Using this formula, 100 ml/kg/day (approximately 4 ml/kg/hr) is given for each of the first 10 kg body weight, 50 ml/kg/day (approximately 2 ml/kg/hr) is given for each kilogram body weight between 11 and 20 kg and 25 ml/kg/day (approximately 1 ml/kg/hr) is given for each kilogram of body weight above 20 kg. The glucose infusion rate for a 50 kg neonate is more than 2.5 times the calculated fluid maintenance rate using this formula (the larger the foal the greater the difference). It is my routine to have foals on the glucose infusion rate for 24 hours or less. By 24 hours, critical foals who can’t receive enteral nutrition are placed on parenteral nutrition and the non-nutritional fluid rate slowed to maintenance levels. [25] Those on enteral nutrition will receive adequate fluids in their milk ration. Thus during the initial 24 hours the neonate may be fluid loaded, often receiving plasma, bolus fluids and glucose at the rate of 8 mg/kg/min in a 10% dextrose solution. But in the most critical foals who are likely to have significant renal compromise, the fluid rate is slowed to this dry maintenance rate (plus parenteral nutrition infusion and medication infusions) within the first 24 hours, sparing the neonate further fluid challenge.

Sodium replacement therapy
It is important to guard against sodium overload of the neonate when formulating maintenance fluids. By combining 5% dextrose in water with sodium containing fluids, fluid and sodium requirements can be delivered independently. Rehydration of all fluid compartments rather than just increasing interstitial fluid is more readily achieved by using sodium dilute fluids. [28] Also, many critically ill neonates tend to have a significant osmolar gap. Adding free water may be important in clearing the unidentified osmolytes.
It is easy to overload a neonate with sodium in part because of their natural sodium conserving physiology and in part because perinatal disease compounds renal sodium handling ability. Sodium overload is a common sequela to indiscriminate fluid therapy with sodium containing fluids. The normal nursing foal receives 1 - 3 mEq Na/kg/day from mare’s milk. The neonate requires about 1 mEq Na/kg/day for growth. The goal of sodium replacement is to limit daily sodium intake to < 3 mEq/kg/day to mimic the normal sodium intake while preventing overload which can cause excessive fluid retention. In the typical size foal, 1 liter of balanced ionic fluids or 1 liter of plasma will deliver the daily sodium requirement. Foals which have been treated with plasma and fluid boluses for hypovolemia during the first 24 hours may not require additional sodium for several days because of the unavoidable sodium loading. Foals receiving 2 gm/kg/day of amino acids in parenteral nutrition formulas may be receiving up to 1 mEq Na/kg/day. Foals will receive additional sodium with drug infusions such as continuous rate infusion of a variety of drugs including inopressors, insulin and antimicrobials as well as bolus infusion of drugs such as antimicrobials and associated saline flushes. Careful attention to sodium intake will help with sodium balance and avoid the fluid overload and accompanying edema secondary to sodium overload.

Some foals have a higher than normal sodium requirement rather than having a tendency for sodium overload. Neonatal nephropathy is a common neonatal problem secondary to hypoxic ischemic disease or SIRS. These foals will have renal sodium wasting as reflected by high sodium clearance and high fractional excretion of sodium. This pathologic renal loss can result in higher than normal sodium requirements in these foals. Clinically, without gastric reflux and without diarrhea, urine is the only source of sodium loss. In cases with neonatal nephropathy sodium can be balanced by matching sodium delivery to urinary losses plus sodium growth requirement (1 mEq/kg/day). Urinary sodium loss can be measured with total urine collection and calculation of daily sodium excretion. Total urine collection requires maintaining a urinary catheter which poses a serious risk of life threatening nosocomial infection or both, cooperation from the patient and intensive management which may not be possible. In cases where total urine collection is not available, sodium requirements can be estimated from urine sodium concentrations and fractional excretion. Although the critical foal with normal renal function requires sodium restriction to prevent sodium overload, foals with renal sodium wasting can have such high losses that they may rapidly become dangerously hyponatremic. It is critical to follow the sodium balance carefully in these cases. Another source of excessive sodium loss is diarrhea. Foals with diarrhea require sodium containing replacement fluids to match their excessive gastrointestinal losses. They should not be sodium restricted.

Potassium replacement therapy
Potassium requirements are difficult to estimate. They will depend on renal losses, anabolic requirements (new cells require large quantities), catabolic potassium release, intracellular shifts associated with cellular glucose transport, modulation of sodium/potassium pump activity by high epinephrine levels and potassium release from sepsis. Any neonate not consuming milk, which is high in potassium, will require supplemental potassium. When delivering a high fluid rate, such as when glucose is being delivered, empirical supplementation with 10-40 mEq/l fluids is usually sufficient. When delivering fluids at the dry maintenance rate, higher concentrations in the range of 20-60 mEq/l will be required. If the patient is allowed to become hypokalemic because of lack of potassium supplementation, very aggressive replacement therapy in the range of 80 – 100 mEq/l may be necessary to “catch up.”
Summary
Fluid therapy is a universally utilized therapeutic modality in critical care patients. To effectively deliver fluids to neonates, an understanding of their fluid physiology is necessary. Neonates, as they make the transition from fetal physiology, have increased capillary filtration and a compliant interstitium producing a large interstitial fluid reserve. This reserve helps the neonate adapt to fluid challenges, serving as a ready source of fluids in times of need but it also frustrates therapeutic fluid administration by damping the effect of intravenous fluid therapy when treating hypovolemia. Additionally it explains the difficulty neonates have handling fluid overload. Successful treatment of hypovolemia requires aggressive volume repletion using 20 ml/kg fluid boluses. Once volemia is restored and ongoing losses replaced, maintenance fluid rates should be conservative to avoid fluid overload. Neonate’s unique sodium handling must also be recognized. Many critical neonates will benefit from sodium restriction whereas others may have high ongoing losses and require careful sodium replacement therapy. Careful attention to fluid therapy formulation will insure positive fluid support without adding to the physiologic stress of the critical neonate.
References
1. Friis-Hansen B. Body water composition in children: Changes during growth and related changes in body composition. Pediatrics 1961; 28: 169-78.
2. Brace RA.
Fetal extracellular fluid and lymphatic function. In: Brace RA, Hanson MA,
Rodeck CH, editors. Fetus and Neonate: Physiology and clinical applications.
Volume 4: Body fluids and kidney function.
3. Brace RA,
Ross MG. Amnionic fluid volume regulation. In: Brace RA, Hanson MA, Rodeck CH,
editors. Fetus and Neonate: Physiology and clinical applications. Volume 4:
Body fluids and kidney function.
4. Brace RA, Gold PS. Fetal whole body interstitial compliance, vascular compliance, and capillary filtration coefficient. Am J Physiol 1984; 247: R800-5.
5. Brace RA.
Fluid Distribution in the Fetus the Neonate. In: Polin RA, Fox WW, editors.
Fetal and Neonatal Physiology, 2nd edition.
6. Brace RA. Thoracic duct lymph flow and its measurement in chronically catheterized sheep fetus. Am J Physiol 1984; 256: H16-20.
7. Gest
8. Brace RA, Cheung CY. Role of catecholamines in mediating fetal blood volume decrease during acute hypoxia. Am J Physiol 1987; 253(4):H927-32.
9. Brace RA. Fetal blood volume responses to acute fetal hemorrhage. Circ Res 1983; 52: 730-4.
10. Brace RA, Cheung CY. Fetal cardiovascular and endocrine responses to prolonged fetal haemorrhage. Am J Physiology. 1986; 251: R417-24.
11. Brace RA. Fetal blood volume responses to intravenous saline solution and dextran. Am J Obstet Gynecol. 1983; 147:777-81.
12. Brace RA,
13. Linshaw MA.
Dilution of the urine. In: Polin RA,
Fox WW, editors. Fetal and Neonatal
Physiology, 2nd edition.
14. Brace RA, Miner LK, Siderowf AD, Cheung CY. Fetal and adult urine flow and ANF responses to vascular volume expansion. American Journal of Physiology. 1988; 255(5):R846-50.
15. Engle WD. Blood pressure in the very low birth weight neonate. Early Human Development. 2001; 62(2):97-130.
16. Lumbers ER,
Gibson KJ, Stevenson KM. Changes in renal function at birth. In: Brace RA,
Hanson MA, Rodeck CH, editors. Fetus and Neonate: Physiology and clinical
applications. Volume 4: Body fluids and kidney function.
17. Gibson KJ, Lumbers ER. Changes in renal function and blood volume in the newborn lamb delivered by caesarean section. Pediatric Research. 1994; 36:506-13.
18. Smith FG, Lumbers ER. Changes in renal function following delivery of the lamb by caesarean section. Journal of Developmental Physiology. 1988; 10:145-8.
19. Rook JS, Braselton WE, Lloyd JW, Shea ME, Shelle JE. Comparison of elemental concentrations in Arabian mare’s milk and commercial milk replacer. Veterinary Clinical Nutrition. 6(2):17-21.
20. Lumbers ER, Hill KJ, Bennett VJ. Poximal and distal tubular activity in chronically catheterized fetal sheep compared to adult. Canadian Journal of Physiology Pharmacology. 1988; 66:697-702.
21. Goldsmith DI, Drunkker A, Blaufoz MD, Edelmann CM, Spitzer A. Hemodynamic and excretory responses to the neonatal canine kidney to acute volume expansion. Am J Physio. 1979; 237:F392-7.
22. Carcillo JA,
23. Palmer JE. When fluids are not enough: Inopressor therapy. In Proceedings of the 8th International Veterinary and Critical Care Symposium, San Antonio, 2002 p. 669-673.
24. Silver M, Comline RS. Fetal and placental O2 consumption and the uptake of
different metabolites in the ruminant and horse during late gestation. Advances
in Experimental Medicine & Biology. 1976;75:731-6.
25. Palmer JE. Practical Approach to Fluid Therapy in Neonates. In Proceedings of the 8th International Veterinary and Critical Care Symposium, San Antonio, 2002 p. 665-8.
26. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823-832.
27. Chesney RW. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1998; 102(2):399-400.
28. Magder LS. Physiologic principles of fluid therapy. Pediatric Critical Care Medicine. 2001;2(3): S4-S9.
|
Table 1. Composition of commonly used intravenous crystalloids fluids |
|||||||
|
|
|||||||
|
Intravenous fluids |
mOsm/l |
mEq/l |
SID |
||||
|
|
|
Na+ |
Cl− |
K+ |
Ca++ |
Mg++ |
|
|
Normosol®-R |
294 |
140 |
98 |
5 |
- |
3 |
53 |
|
Plasmalyte® |
298 |
140 |
98 |
5 |
- |
3 |
53 |
|
Lactated Ringer's solution |
275 |
130 |
109 |
4 |
3 |
- |
31 |
|
0.9% NaCl |
308 |
154 |
154 |
- |
- |
- |
0 |
|
5% Dextrose in water |
253 |
- |
- |
- |
- |
- |
0 |